Senator Ron Johnson and the Department of Defense Whistleblowers pertaining to the covid vaccine
https://www.ronjohnson.senate.gov/services/files/2266AE5A-027A-42F2-90E1-D4430F32BF37
On August 18th, Ron Johnson sent a letter to to the DOD, the FDA, and the CDC regarding a whistleblower investigation pertaining to Comirnity labeled vaccines sent to the Coast Guard in Alaska back in June, 2022. From the link above is the following screen shots, as the PDF sent by Senator Johnson is not able to be copied or pasted. For the full read, see the link above.
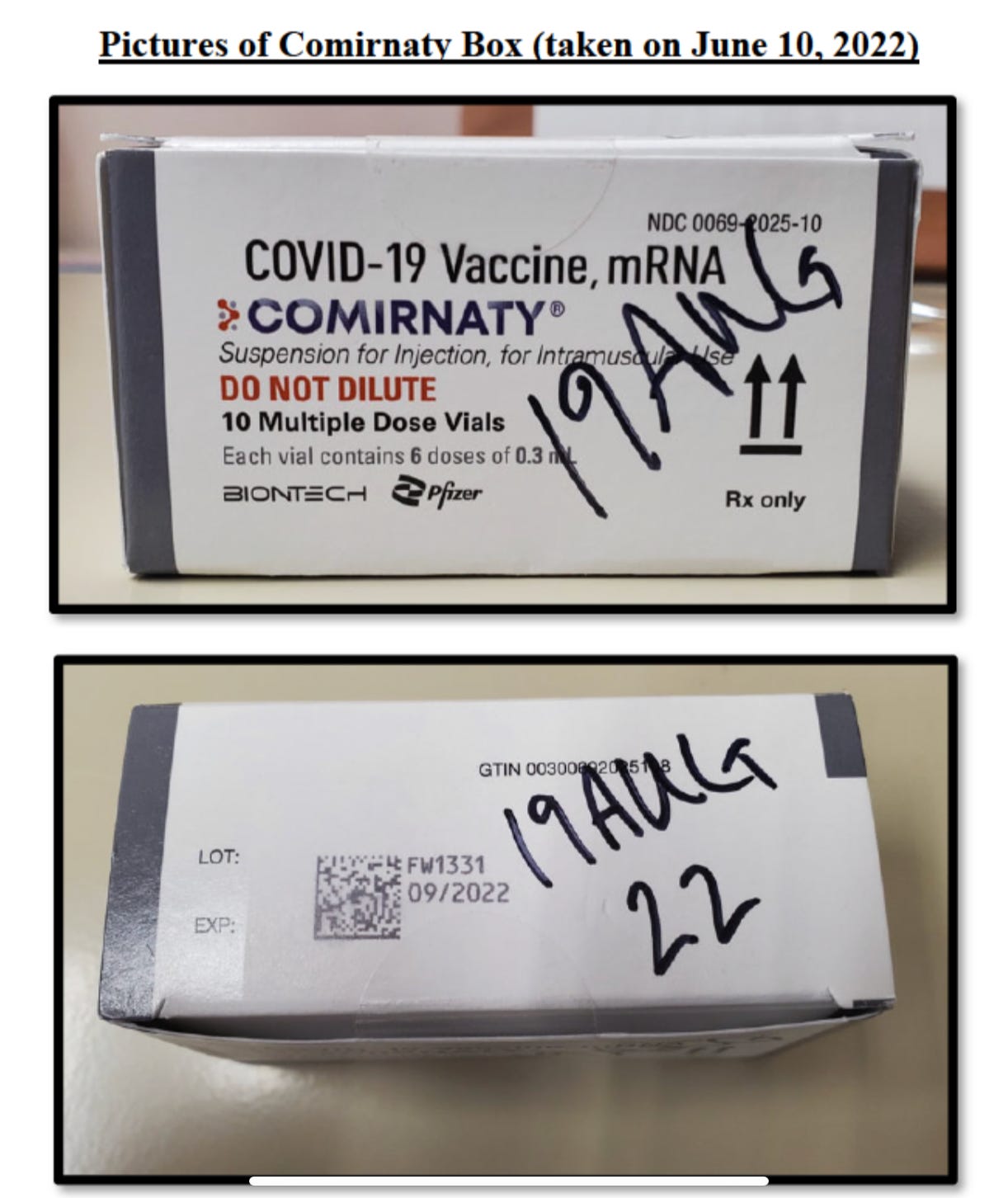
Here are the photos, you can see they are lot number FW1331, with an expiration date of 9/2022.
In this next photo, the date hand written ON the box is August 19th, when the vials themselves state they are good through 9-30-2022.
Given that Lt. Coppin had never received any Comirnity vials on his base before, and the box instructions of a hand written expiration date seemed out of the normal, he decided to investigate WHERE these Comirnity vials came from. Now keep in mind here, the Coast Guard has been EXTREMELY “compliant” with the vaccine mandates, and nearly their entire branch has received the covid vaccine. So this was not some act of subterfuge by a group who has fought back against vaccine mandates. Quite the opposite actually. This is a heavily vaccinated branch of government.
The chain of custody of lot number FW1331 goes like this: the Alaska base medical staff said they came from Ft. Dietrich, Maryland. When Coppin contacted Ft. Dietrich, they informed him that the vials came from the Pfizer plant in Kalamazoo, Michigan. Coppin then called Pfizer directly, and Pfizer informed him that THIS lot number, FW1331, was manufactured in FRANCE on January 28th, 2022, and have an expiration date of 12-31-2022.
There is a teensy little problem with the answer that Pfizer gave him: France is NOT an approved manufacturing location for Comirnity vaccine. In the FDA biologics license application and supplemental approval letter, given to Pfizer by the FDA, dated on December 30th, 2021, it states that “the approved Comirnity vaccine which is a Tris/Sucrose 30mcg formulation” is to be manufactured at the “Pfizer Manufacturing Belgium NV, Purrs Belgium facility”. Any vaccine manufactured outside of the BLA approved site has no oversight for safety, manufacturing, and processing, and could possess a risk to the recipients of the unsupervised production vaccine.
So further down the rabbit train here, because in total we have NINE whistleblowers from the DoD here, 1st Lieutenant Army Commissioned Mark Bashaw questioned the validity of the contents in those vials being Comirnity vaccines. Bashaw downloaded the EUA vaccine lot number database, and FW1331 was listed under the EUA vaccine lots. Which, Comirnity is NOT EUA, remember, that one was allegedly given “full FDA approval” in August 2021, so it would NOT be under EUA use. So if this vial lot is indeed Comirnity, with that lot number, it should not be on the EUA list, as it is the approved brand use per the FDA a year ago.
Here is the screenshot of the concerns the whistleblowers had:
I highly recommend you copy and paste the link above at the beginning of the post, and read the enclosure that was written by and signed by the 9 whistleblowers. Their concerns are worth the read.





Huge sigh...several of them as I read this, particularly when I got to No. 4 on the Enclosure. I had to stop. I will go back and finish reading, Dr. Brown, but this is not my country. I had a "sneaking suspicion" about that for a long time, but it has been proven over the last 2.5 years. All of it happened, IMO, because of the revealed craven, sanctimonious nature of the "average 'American'". All this? Over the chance of getting a respiratory infection?
And in typical fashion: no response yet from Wolinsky or the CDC. “No response is a response” Cards are falling. Hope everyone at CDC has sold their shares of pharma.