Remdesivir has become a household name thanks to the covid pandemic. Remdesivir was not a new drug though, and the history of this antiviral medication is highly concerning. I will also state that I firmly believe the use of Remdesivir led to kidney failure in more than a few people and patients I know. I also believe it contributed to the death of 3 people that I have knowledge of. I am not pro-Remdesivir for the reasons stated below, and in follow up posts over the next couple of days.
Remdesivir is made by Gilead sciences. Back in 2017, Gilead partnered with the University of North Carolina Gillings school of public health to what? STUDY BAT CORONAVIRUSES. And how to treat them with antivirals. Who did Gilead partner with? Dr. Ralph Baric, one of the Virologists at UNC, who is ALSO connected to Wuhan Institute of Virology and to Dr. Anthony Fauci. Baric was instrumental in making sure that the “lab created/lab leak” theory of Covid was squashed early in 2020. https://sph.unc.edu/sph-news/gillings-researchers-receive-6m-grant-to-fight-infectious-disease/
Look at how they were already putting zoonotic jumping into the “mainstream” narrative in 2017?!?! To quote the above article “To date, there are no approved therapies to treat any kind of CoV infection. Coronaviruses are of special concern to public health practitioners because they can jump, without warning, from animals into the human population, and they tend to spread rapidly. The elderly are especially vulnerable. Emerging CoV represent a significant and ongoing global health threat,” said Baric. “For the first time, our studies are providing potent treatment options designed not only to protect individuals from life-threatening coronavirus infections but also to block transmission patterns in high-risk settings. The UNC-Gilead partnership provides an outstanding model designed to protect the public against current and future emerging virus outbreaks.” Other universities were partnered in this as well. Vanderbilt and University of Texas.
Moving on. Back to Remdesivir.
Remdesivir being owned by Gilead had a direct interest in being the “antiviral of choice” (AKA make lots of money) during the covid pandemic. Remdesivir was NOT a new drug. It originally came out in 2009 as a treatment for Hepatitis C and RSV. When you look at studies OF Remdesivir though, there is no mention of what that drug did in 2009. That data has been wiped. It only discusses Ebola trials in later years. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7202249/.
This would lead one to believe that SOMETHING did not work right back in 2009. Either the drug was a huge flop and did not work in treating Hepatitis C and RSV, or bad things happened when the drug trials happened. Back on the shelf it went.
In 2014, Remdesivir was again put on trial, this time for Ebola virus. Per the above linked NCBI clinical trial, “Remdesivir was included in a randomized, controlled trial of Ebola virus therapeutics in patients within the Democratic Republic of the Congo (NCT02818582); however, midstudy primary analyses found remdesivir INFERIOR to the antibody (monoclonal antibodies) MAb114 and REGN-EB3, with respect to mortality (more people died with Remdesivir than monoclonals), and the Remdesivir intervention arm was terminated. Reported adverse events related to remdesivir include hypotension, elevated creatinine and aspartate aminotransferase plasma levels (a suggestive marker for impaired kidney or liver function, respectively) in remdesivir-treated patients compared to either antibody based therapeutic arms. Remdesivir was inferior against EBOV based on efficacy compared to antibody therapy. This NCBI article mentions NOTHING about the treatment study outcomes.
But this study does. https://www.nejm.org/doi/full/10.1056/NEJMoa1910993
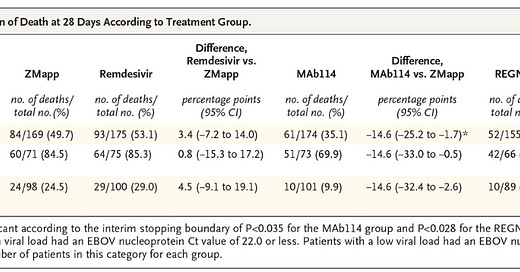
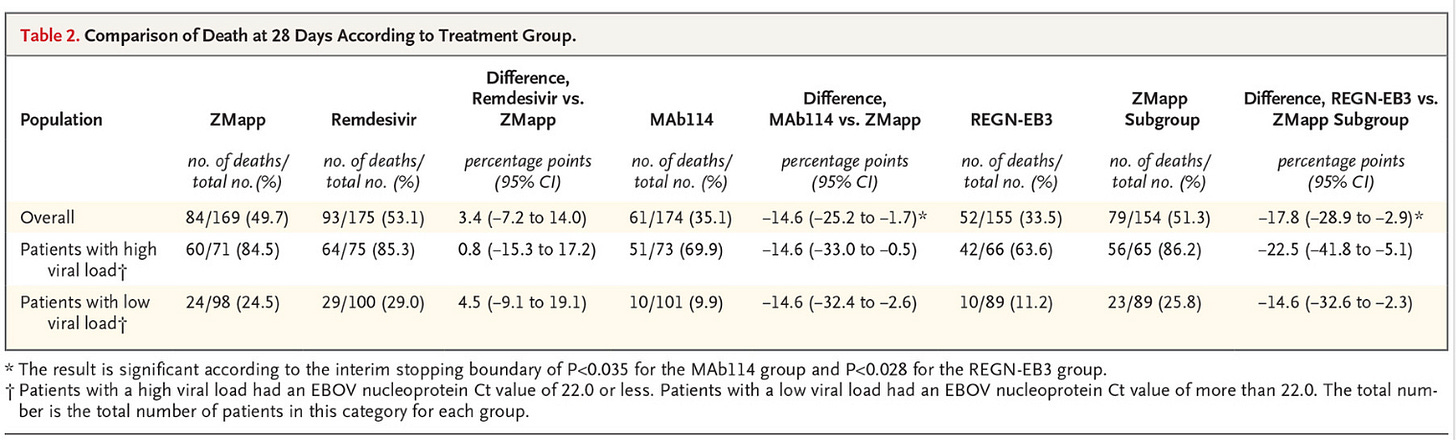
In this 2018-2019 study, Remdesivir was again evaluated for Ebola. A total of 673 patients were enrolled in a trial that compared Remdesivir & ZMAPP (both experimental drugs for Ebola), versus 2 different monoclonal antibodies in the treatment of Ebola. Results table is below, but we can conclusively interpret that Remdesivir had THE HIGHEST DEATH RATE in patient with high AND low viral load of Ebola. The second highest death rate was the experimental ZMAPP drug. The most successful treatment for both high and low viral loads was monoclonal antibodies. Patients given the monoclonal antibodies had a 14-23% HIGHER SURVIVAL versus the ZMAPP, and those numbers are even higher when compared to Remdesivir. Remdesivir was the deadliest treatment modality in this trial. The side effects of Remdesivir were difficult to evaluate in the trial, because the Ebola virus in and of itself is horrid, highly fatal, and it would be difficult to determine medication side effect versus viral illness effects. Perfect virus to trial a drug on, if you don’t want to know side effect efficacy, or if you want to cover up potential drug deaths. It is nearly impossible to know if the virus or the drug killed them.
So back on the shelf went Remdesivir. Trialed for 3 viruses, and it was not successful.
Until June 2020. Dr. Anthony Fauci advocated that Remdesivir should be trialed in covid-19 patients as “compassionate care” trials. THIS TRIAL was used as the decision making trial for use of Remdesivir as “the drug of choice” in treating covid. The “right to try” is the same thing as compassionate care trials. He brought back Remdesivir AGAIN, for a THIRD go round, an antiviral already shown to cause liver and kidney problems, a medication shown to have poor efficacy in treating viruses. And in doing so, ALL other medications were removed from right to try. Hydroxychloroquine. Ivermectin. Those were deemed “illegal” to use in compassionate/right to try care. If you were approved for the Remdesivir treatment, the rules were: “patients were required to have a creatinine clearance above 30 ml per minute and serum levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) less than five times the upper limit of the normal range, and they had to agree not to use other investigational agents for Covid-19.
https://www.nejm.org/doi/full/10.1056/NEJMoa2007016
In total, 53 patients were enrolled in this trial. Some got 10 days of Remdesivir, some only got 5-9 days of the medication. The results of the “compassionate care trial”: 13% of patients died in the 61 person trial. 8% had to stop the trial due to liver/kidney/allergic reaction to Remdesivir. 60% reported ADVERSE EVENTS from the medication. “Summary of Adverse Events.
A total of 32 patients (60%) reported adverse events, the most common adverse events were increased hepatic enzymes, diarrhea, rash, renal impairment, and hypotension. In general, adverse events were more common in patients receiving invasive ventilation. A total of 12 patients (23%) had serious adverse events. The most common serious adverse events — multiple-organ-dysfunction syndrome, septic shock, acute kidney injury, and hypotension”.
Other notable conclusions/findings from this trial: “To date, no therapy has demonstrated efficacy for patients with Covid-19”. “Unfortunately, our compassionate-use program did not collect viral load data to confirm the antiviral effects of remdesivir or any association between baseline viral load and viral suppression, if any, and clinical response. Moreover, the duration of remdesivir therapy was not entirely uniform in our study”.
Soooooo dosages were not the same, days on the med were not the same……..
Also note, who did this study…..Gilead……the company who MAKES the drug.



I'm putting a link to this post and Part 2 on my page... I am VERY concerned about this drug, and the plans to give it to infants!!! Thanks, I just found you by searching for a history of the drug. I'm on Substack, too. Good work, and thanks!